By: Cassie Penn, Client Manager, Lighthouse Lab Services/Vachette
As the clinical laboratory advances, we are seeing molecular testing become more available with a wide array of different tests available to physicians and patients. With the increase of molecular tests, we are seeing changes in payer policies. Molecular testing is becoming the gold standard when it comes to laboratory testing; however, payors still see the testing as investigational or not medically necessary.
Understanding Medicare Policy
CMS relies on a network of Medicare Administrative Contractors (MACs) to serve as a contact between the Medicare FFS program and healthcare providers. MACs are multi-state, regional contractors responsible for administering both Medicare Part A and Medicare Part B claims. Currently, there are seven MACs. They are: Noridian, Wisconsin Physicians Service Government Health Administrators (WPS), Novitas, Palmetto, National Government Services (NGS), CGS, and First Coast.
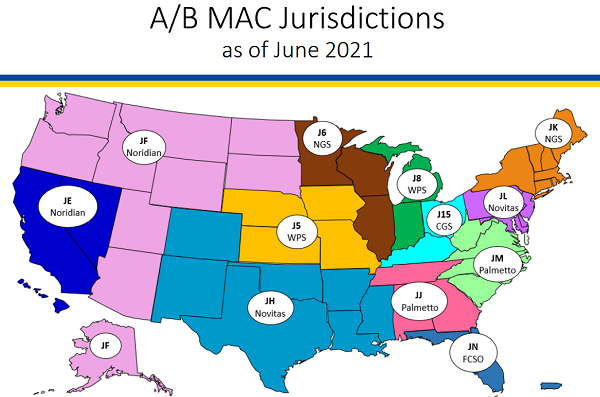
The Seven Current MAC Jurisdictions. (Courtesy CMS)
Each MAC creates medical policies known as Local Coverage Determinations (LCDs). Articles are then linked to the LCD. These LCDs discuss policy on billing and coding guidelines. These guidelines have to do with CPT and Diagnosis codes. Commercial payors like Blue Cross Blue Shield and United Healthcare tend to follow MAC policies. However, it is important to know each MAC jurisdiction and commercial payors may be a little different.
When analyzing a molecular test, it is important to understand Medicare reimbursement, medical policy, and payor mix to determine the return on investment (ROI) for the given test.
For example, Novitas has an Article A58795 Billing and Coding: Genetic Testing for Cardiovascular Disease that was recently updated to state: Tier 2 CPT code 81046 is not appropriate to report for cardiovascular genetic testing because there are no genes associated with CPT code 81406 that meet the ClinGen evidence standards.
What Does This Mean?
If a molecular laboratory has a genetic cardiology panel with a gene that has 81406 as a CPT code, Novitas MAC will not pay for this CPT code. Currently, Novitas and First Coast are the only MACs with this LCD/NCD policy. However, most MACs will probably follow suit. This usually means commercial payors will also develop a policy on this CPT code regarding genetic testing in a cardiology panel. We see the MACs updating policy regularly. For example, the links below were recently updated:
MAC: Novitas
- Billing and Coding: Biomarkers Overview (A56541)
- Billing and Coding: Pharmacogenomics Testing (A58801)
- Genetic Testing for Cardiovascular Disease (L39082)
- Billing and Coding: Genetic Testing for Cardiovascular Disease (A58795)
- Billing and Coding: Biomarkers for Oncology (A52986)
As a busy lab leader, thinking about complicated billing issues is the last thing you want to deal with. However, it is important to have a general idea of how medical policy works and how it will affect reimbursement of the current testing or new testing you will be performing. When completing an ROI on a new test, it is important to have a good understanding of how payment will be affected. Laboratory leadership does not want to bring in a test that will not be reimbursed by any payors. It is important to remember just because you billed for the test does not mean you will get paid for it.
There are multiple steps to the billing process. The laboratory needs to have a good process in place to ensure quality data is collected upfront to pass to the biller. Important steps in the process include:
- Clear options on the requisition
- Clear process that occurs in a clear sequence
- Prepare for denials form insurance
- Have clear documentation for an appeal to the denial
- Understand it may still get denied
- Understand payor mix
As molecular testing evolves, it will be interesting to see how payors evolve with medical policies for molecular testing reimbursement. Molecular tests offer a lot to the medical diagnosis of a patient. However, payers have not moved at the same pace and may still deny molecular tests that are beneficial to the patient.
If you’re looking for assistance in better understanding Medicare policy for molecular testing reimbursement or how to develop your test menu, don’t hesitate to reach out to us directly!

nice discussion on the increase in molecular testing. More and more payers are mandating that the ordering physician has an ‘established’ relationship with the patient and knows precisely which tests are being ordered and how they will be used in the treatment of the patient. This documentation plays a significant role in after payment audits and thus the abillity to keep the funds once paid.
Thank you, Brenda. Glad you found the article to be informative. This is definitely an issue our clients are having to navigate with increasing frequency and will be something to monitor in the coming years.